THE LIME PROCESS
Limestone is a naturally occurring and abundant sedimentary rock consisting of high levels of calcium and/or magnesium carbonate, and/or dolomite (calcium and magnesium carbonate), along with minerals.
Lime production begins by extracting limestone from quarries and mines.
Processed stone is transported by conveyor belt to the lime kilns.
Quicklime can be processed into hydrated lime by adding water to the crushed lime, and then classifying the hydrated lime to ensure it meets customer specifications before it is transported.
Information update 2013 - Data, information and photos courtesy of:
National Lime Association ("Lime Facts" bulletin),
U.S. Geological Survey ("Mineral Commodity Summaries")
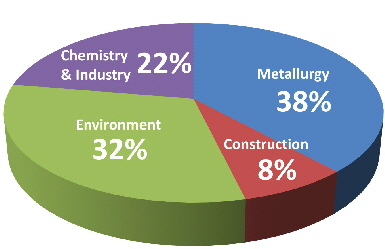
LIME SOLD AND USED BY PRODUCERS IN THE UNITED STATES, BY USE
The term “Lime”![]()
The term "lime" refers primarily to six chemicals produced by the calcination process and hydration, where necessary.
- CaO quicklime
- CaO + MgO dolomitic quicklime
- MgO dead burned dolomite
- Ca(OH)2 hydrated lime
- Ca(OH)2 + MgO type N dolomitic hydrate
- Ca(OH)2 + Mg(OH)2 type S dolomitic hydrate
The last 50 years have seen tremendous growth in lime output despite some significant decreases during poor economic times and some major market shifts.
Changing technology, economics and environmental regulations have played significant roles in some lime markets. The steel industry is the best example of where a change in technology - the replacement of open-hearth furnaces with basic oxygen furnaces - resulted in a dramatic change in the quantity and types of lime used.
Lime is used in environmental, metallurgy, construction, chemical/industrial applications, among many others. The fastest growing use of lime is in environmental applications, where lime is used to comply with air, drinking water, wastewater, and solid waste regulations.
However, the largest single use of lime remains steel manufacturing, where it is used to remove impurities. Lime is also used to produce other metals. In construction, the main use of lime is in soil stabilization for roads, earthen dams, airfields, and building foundations. As an additive in asphalt, lime improves its cohesion, reduces stripping, and delays the aging process. Lime is also a key ingredient in mortar and plaster. Additional chemical and industrial uses of lime include the manufacturing chemicals and production of precipitated calcium carbonate.



CaCO3
CaO
Ca(OH)2